
English - 2
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Model / Serial Number:
Date of Purchase:
Midmark Authorized Service
Company:
Dealer:
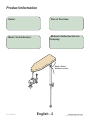
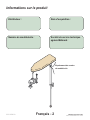
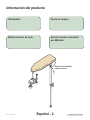
Product Information
Model / Serial
Number Location

English - 3
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Table of Contents
Important Information
Product Information ....................................................................................................2
Product Registration ................................................................................................... 4
Transportation / Storage Conditions ...........................................................................4
Operating Conditions ..................................................................................................4
Disposal of Equipment ................................................................................................4
Authorized Representatives .......................................................................................4
Important Information ................................................................................................6
Intended Use ..............................................................................................................7
Requirements .............................................................................................................7
Safety Instructions ...................................................................................................... 7
Set Up
Set Up Instructions .....................................................................................................8
Operation
Rotational Positioning .................................................................................................9
Height Adjustment ....................................................................................................10
Maintenance
Calling for Service .................................................................................................... 11
Cleaning ................................................................................................................... 11
Preventative Maintenance ........................................................................................ 11
Warranty Information
Limited Warranty ......................................................................................................12

English - 4
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Disposal of Equipment
At the end of product life, the chair, accessories, and other consumable goods may have become
contaminated due to normal medical use. Consult local codes and ordinances for proper disposal
of equipment, accessories and other consumable goods.
Product Registration
To register your product, go to www.midmark.com
Operating Conditions
Ambient Temperature Range: ..................................... +10°C to +40°C (+50°F to +104°F)
Relative Humidity........................................................ 30% to 75% (non-condensing)
Altitude........................................................................ 3000m or less
Transportation / Storage Conditions
Ambient Temperature Range: ..................................... +5°C to +38°C (+41°F to +100°F)
Relative Humidity........................................................ 10% to 90% (non-condensing)
Atmospheric Pressure ................................................ 500hPa to 1060hPa (0.49atm to 1.05atm)
Authorized Representatives
Customers in the EU should direct all questions, incidents, and complaints to Midmark’s Authorized
Representative listed below.
CEpartner4U
Esdoornlaan 13
3951 DB Maarn, The Netherlands
Phone: +31 343 442 524
Fax: +31 343 442 162
Customers in the UK should direct all questions, incidents, and complaints to Midmark’s UK Responsible Person listed
below.
UKCApartner4U Ltd.,
7 CAMPION WAY, BINGHAM, NOTTINGHAM NG13 8TR, UK.
www.UKCApartner4U.co.uk
Phone : +44 7905 384429
Customers in Australia should direct all questions, incidents, and complaints to Midmark’s Sponsor listed below.
ICONA Pty Ltd.
1A/2A Westall Road
Clayton, Victoria 3168
Phone: 1300 442 662
WARNING
In the event of any serious incident in relation to the device, please contact
Midmark and the appropriate competent authority.

English - 5
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Authorized Representatives - continued
Customers in India should direct all questions, incidents, and complaints to Midmark’s Authorized Representative listed
below.
Midmark India
Kalpataru Point, Unit No. 12, 1st Floor Kamani road, Sion (E)
Mumbai, 400022
Tel:+91 22 4915 3000
Fax:+91 22 4915 3100
Customers in Saudi Arabia should direct all questions, incidents, and complaints to Midmark’s Authorized Representative
listed below.
Attieh Medico Ltd
Al Nakheel Dist. II
P.O. Box 116105
Jeddah (East)
21391
Saudi Arabia
Tel: + 966 2 286 4707
Fax: + 966 2 286 4744
Customers in the United Arab Emirates should direct all questions, incidents, and complaints to Midmark’s Authorized
Representative listed below.
GULF DRUG LLC.
Al Barsha,
Dubai, UAE
Phone: +971 4 501 4000
Fax: +971 4 501 4100
Customers in Hong Kong should direct all questions, incidents, and complaints to Midmark’s Authorized Representative
listed below.
Associated Medical Supplies Company LTD.
Room 1201-1202, 12/F, Fo Tan Industrial Center
26-28 Au Pui Wan Street, Fo Tan, Shatin
New Territories, Hong Kong
Phone: +852 2604 9389
fax: +852 2694 0866
Customers in Qatar should direct all questions, incidents, and complaints to Midmark’s Authorized Representative listed
below.
Tadmur Trading
Midmac Roundabout
Opposite Al Mana Petrol Station
Doha, Qatar
Tel: +974.44337000
Fax: +974.44337100
Customers in Israel should direct all questions, incidents, and complaints to Midmark’s Authorized Representative listed
below.
Medtechnica Ltd.
HaTnufa St 7
Petah Tikva, Israel
Tel: +972 3.925.4040
Fax: +972 3.924.9977

English - 6
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Catalogue Number
Manufacturer
Serial Number
WARNING
Indicates a potentially hazardous situation which could result in serious injury.
Important Information - Safety Symbols
Caution
Indicates a potentially hazardous situation which may result in minor or moderate injury. It
may also be used to alert against unsafe practices.
Equipment Alert
Indicates a potentially hazardous situation which could result in equipment damage.
Proper shipping orientation
Fragile
Keep dry
Note
Amplifies a procedure, practice, or condition.
Symbol Glossary
Medical Device

English - 7
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Intended Use
To support the patient’s arm during procedures on the upper extremities.
Safety Instructions
WARNING
No modification of this equipment is allowed.
WARNING
Do not sit on Armboard or Armboard pad.
WARNING
Do not operate chair with this accessory installed or press any button during a procedure.
WARNING
Do not use Armboard as a gripping surface during ingress egress.
Requirements
Depending on chair model, a back mounted accessory rail may need to be installed.

English - 8
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Set Up
WARNING
Do not use with any other accessories on same side of chair. Assure all positions are solidly
locked to prevent sudden movement during procedure.
Caution
Always support Armboard assembly when changing positions on it.
A) Loosen knob.
B) Slide swivel block onto rail to desired position.
C) Tighten knob.
D) Position leg assembly in location on leg brace and insert pin assembly.
E) Install cushion.
Equipment Alert
Place chair in desired position before mounting 9A82001 Special Procedures Armboard.

English - 9
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Operation - Rotational Positioning
A) Supporting Armboard, loosen T-handle.
B) Rotate Armboard to desired position.
C) Tighten T-handle.

English - 10
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
A) Supporting Armboard, loosen T-handle.
B) Raise or lower Armboard to desired position.
C) Tighten T-handle.
D) Loosen knob.
E) Position lower leg in desired position.
F) Tighten knob.
Operation - Height Adjustment

English - 11
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Maintenance
Calling for Service
If service is required, contact your authorized Midmark dealer.
To contact Midmark Versailles Tech Service directly:
1.800.MIDMARK (1.800.643.6275)
8:00 am until 7:00 pm Monday - Thursday (ET) [excluding standard US holidays]
8:00 am until 5:00 pm Friday (ET) [excluding standard US holidays]
www.midmark.com
Cleaning
Armboard Pad
Wash your Armboard weekly with a mild liquid soap and water mixture, rinse with clear water and dry
completely to remove disinfection cleaner build-up.
Disinfect your Armboard with a solution of standard bleach and water mixed 1 in 10 (10%) or chlorine based
cleaners. Follow this with a clear water rinse and thorough drying of material. See current CDC Guideline for
Disinfection & Sterilization in Healthcare Facilities.
To minimize damage caused by disinfectant cleaner residue build-up, do not allow disinfectants to pool on
the Armboard surface. Once the approved contact time has been obtained, remove and dry excess liquid
remaining on the surface.
Detailed care and maintenance instructions are included with your product. This information is also available
on www.midmark.com in the Technical library under the User Information tab for your product.
Painted Metal / Plastic Surfaces
Clean the painted metal and plastic surfaces weekly using a clean soft cloth, and mild cleaner.
Preventative Maintenance
Periodically inspect the following areas:
• All fasteners should be in place and tightened securely.
• All mechanical functions should operate properly.
Note
Model / serial number information is required when calling for service.
Equipment Alert
The Armboard is resistant to most medicinal-type stains, but may be
damaged by solvents and dyes. Immediately remove any fluids spilled
on the Armboard.

English - 12
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
SCOPE OF WARRANTY Midmark Corporation (“Midmark”) warrants to the original retail purchaser that it will, at Midmark’s option, repair or
replace components of the domestic and international medical products manufactured by Midmark (except for components not warranted
under “Exclusions”) that are defective in material or workmanship under normal use and service. The sole remedy under this limited warran-
ty is the repair or replacement, at Midmark’s option, of the applicable components. This limited warranty shall only apply to defects that are
reported to Midmark within the applicable warranty period and which are determined to exist upon examination by Midmark,. This warranty
extends only to the original retail purchaser of a product and is not transferable or assignable. Replacement components or products may
Midmark warrants to the original retail purchaser that during the applicable warranty period it will repair or replace software contained
within the products manufactured by Midmark (except for those not warranted under “Exclusions”) if: (1) the media on which the software is
furnished exhibits defects in material or workmanship under normal use; or (2) the software does not substantially conform to its published
APPLICABLE WARRANTY PERIOD The applicable warranty period, measured from the date of invoice to the original retail purchaser of
the product and shall be one (1) year for all warranted products and components. QuickClean® Ultrasonic Cleaners are warranted for a
period of three (3) years.
OBTAINING WARRANTY SERVICE Warranty service must be obtained through either Midmark or an authorized dealer in the Midmark
product line for which warranty service is requested. Midmark may be contacted for warranty service inquiries or issues via email at
midmark.com; by phone at 1.800.MIDMARK or by mail to Midmark Corporation, 60 Vista Drive, Versailles, Ohio 45380. It is the retail pur-
chaser’s obligation to arrange for delivery of a product to Midmark or one of its authorized dealers for warranty service, which delivery shall
be at retail purchaser’s expense. It is also the retail purchaser’s obligation to comply with the warranty service instructions provided either
by Midmark or its authorized dealer. The retail purchaser must provide Midmark with completed warranty registration information within
EXCLUSIONS: This limited warranty does not cover and Midmark shall not be liable for the following:
(1) Defects, damage or other conditions caused, in whole or in part, by misuse, abuse, negligence, alteration, accident, freight damage,
negligent storage, tampering or failure to seek and obtain repair or replacement in a timely manner;
(2) Products which are not installed, used, and properly cleaned and maintained as required or recommended in the Midmark “Installation”
and electrical requirements;
(3) Products considered to be of a consumable or sterile nature;
(4) Accessories or parts not manufactured by Midmark;
(5) Charges by anyone for adjustments, repairs, replacement parts, installation or other work performed upon or in connection with such
products which are not expressly authorized in writing in advance by Midmark;
(6) Costs and expenses of routine maintenance and cleaning; and
(7) Representations and warranties made by any person or entity other than Midmark.
(8) Matching of color, grain or texture except to commercially acceptable standards;
(10) Custom manufactured products;
(12) Products that would otherwise by covered under this limited warranty, but are acquired: (i) from a person or entity that is not Midmark
or one of its authorized dealers; or (ii) from a Midmark dealer that is not authorized to sell the product at issue in the geographic territory
where the purchaser is located, or is not authorized to sell the product at issue within the medical market.
SOFTWARE; WITH RESPECT TO SOFTWARE THAT IS A PRODUCT OR COMPONENT THEREOF, MIDMARK DOES NOT WARRANT
THAT THE SOFTWARE: (1) IS ERROR FREE; (2) CAN BE USED WITHOUT PROBLEMS OR INTERRUPTIONS; OR (3) IS FREE FROM
VULNERABILITY TO INTRUSION OR ATTACK BY VIRUSES OR OTHER METHODS.
EXCLUSIVE REMEDY; CONSEQUENTIAL DAMAGES DISCLAIMER MIDMARK’S ONLY OBLIGATION UNDER THIS LIMITED WARRAN-
TY IS THE REPAIR OR REPLACEMENT OF DEFECTIVE PARTS. MIDMARK SHALL NOT BE LIABLE FOR AND HEREBY DISCLAIMS
ANY DIRECT, SPECIAL, INDIRECT, INCIDENTAL, EXEMPLARY OR CONSEQUENTIAL DAMAGES OR DELAYS, INCLUDING, BUT
NOT LIMITED TO, DAMAGES FOR LOSS OF PROFITS OR INCOME, LOSS OF USE, LOSS OF DATA, DOWNTIME, COVER AND
EMPLOYEE OR INDEPENDENT CONTRACTOR WAGES, PAYMENTS AND BENEFITS. THIS DISCLAIMER SHALL SURVIVE ANY
FAILURE OR ASSERTED FAILURE OF THE ESSENTIAL PURPOSE OF THIS LIMITED WARRANTY OR ITS REMEDIES SPECIFIED
HEREIN. WARRANTY DISCLAIMER THIS WARRANTY IS MIDMARK’S ONLY WARRANTY AND IS IN LIEU OF ALL OTHER WARRAN-
TIES, EXPRESS OR IMPLIED. MIDMARK MAKES NO IMPLIED WARRANTIES OF ANY KIND INCLUDING ANY IMPLIED WARRANTIES
OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. THIS WARRANTY IS LIMITED TO THE REPAIR OR REPLACE-
MENT OF DEFECTIVE PARTS.
STATUTE OF LIMITATIONS No action may be brought against Midmark for breach of this limited warranty, an implied warranty, if any, or for
any other claim arising out of or relating to the products, more than ninety (90) days following expiration of the limited warranty period.
the products.
Midmark® + Ritter® Medical Equipment (Tables, Chairs, Physician
Seating, Lights, Workstations and Sterilizers) Limited Warranty

Midmark Corporation
60 Vista Drive
Versailles, OH 45380 USA
+1.800.643.6275
+1.937.526.3662
www.midmark.com
TP202 20-42-FO-00014 Rev A2 C2169

Deutsch - 2
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Modell-/Seriennummer:
Kaufdatum:
Midmark-Vertragshändler:
Händler:
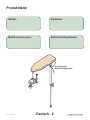
Produktdaten
Anordnung der
Modell-/Seriennummer

Deutsch - 3
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Inhaltsverzeichnis
Wichtige Informationen
Produktdaten ..............................................................................................................2
Produktregistrierung ...................................................................................................4
Richtlinien für Transport und Aufbewahrung ...............................................................4
Betriebsbedingungen ..................................................................................................4
Geräteentsorgung .......................................................................................................4
Autorisierte Repräsentanten .......................................................................................4
Wichtige Informationen ............................................................................................... 6
Bestimmungsgemäße Verwendung............................................................................7
Anforderungen ............................................................................................................7
Sicherheitshinweise ....................................................................................................7
Setup
Setup-Hinweise ..........................................................................................................8
Bedienung
Rotationspositionierung .............................................................................................. 9
Höheneinstellung ......................................................................................................10
Wartung
Kundendienst ............................................................................................................ 11
Reinigung ................................................................................................................. 11
Vorbeugende Wartung .............................................................................................. 11
Garantieinformationen
Eingeschränkte Garantie .......................................................................................... 12

Deutsch - 4
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Geräteentsorgung
Der Stuhl, dessen Zubehör sowie andere Verbrauchsgüter können am Ende des Produktlebenszyklus durch
den normalen medizinischen Betrieb verunreinigt sein. Die örtlichen Verordnungen und Bestimmungen zur
ordnungsgemäßen Entsorgung von Geräten, Zubehör und anderen Verbrauchsgütern beachten.
Produktregistrierung
Das Produkt hier registrieren: www.midmark.com
Betriebsbedingungen
Umgebungstemperaturbereich ................................... +10 °C bis +40 °C (+50 °F bis +104 °F)
Relative Luftfeuchtigkeit ............................................. 30 % bis 75 % (nicht kondensierend)
Höhe ........................................................................... 3000 m oder weniger
Richtlinien für Transport und Aufbewahrung
Umgebungstemperaturbereich ................................... +5 °C bis +38 °C (+41 °F bis +100 °F)
Relative Luftfeuchtigkeit ............................................. 10 % bis 90 % (nicht kondensierend)
Luftdruck ..................................................................... 500 hPa bis 1060 hPa (0,49 atm bis 1,05 atm)
Autorisierte Repräsentanten
Kunden in der EU sollten alle Fragen, Angaben zu Vorfällen oder Beschwerden an den folgenden
autorisierten Vertreter von Midmark richten.
CEpartner4U
Esdoornlaan 13
3951 DB Maarn, Niederlande
Telefon: +31 343 442 524
Fax: +31 343 442 162
Kunden aus dem Vereinigten Königreich richten bitte alle Fragen, Vorfälle und Beschwerden an den unten genannten
Ansprechpartner von Midmark im Vereinigten Königreich
UKCApartner4U Ltd.,
7 CAMPION WAY, BINGHAM, NOTTINGHAM NG13 8TR, UK.
www.UKCApartner4U.co.uk
Telefon : +44 7905 384429
Kunden in Australien sollten alle Fragen, Vorfälle oder Beschwerden an den folgenden Sponsor von
Midmark richten.
ICONA Pty Ltd.
1A/2A Westall Road
Clayton, Victoria 3168
AUSTRALIEN
Telefon: +61 1300 442 662
WARNUNG
Bei einem schwerwiegenden Vorfall im Zusammenhang mit dem
Gerät kontaktieren Sie bitte Midmark und die zuständige Behörde.

Deutsch - 5
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Autorisierte Repräsentanten - Fortsetzung
Kunden in Indien sollten alle Fragen, Vorfälle oder Beschwerden an den folgenden autorisierten Vertreter von Midmark
richten.
Midmark India
Kalpataru Point, Unit No. 12, 1st Floor Kamani road, Sion (E)
Mumbai, 400022
Tel.: +91 22 4915 3000
Fax: +91 22 4915 3100
Kunden in Saudi-Arabien sollten alle Fragen, Vorfälle oder Beschwerden an den folgenden autorisierten Vertreter von
Midmark richten.
Attieh Medico Ltd
Al Nakheel Dist. II
P.O. Box 116105
Jeddah (East)
21391
Saudi-Arabien
Tel.: + 966 2 286 4707
Fax: + 966 2 286 4744
Kunden in der Vereinigten Arabischen Emiraten sollten alle Fragen, Vorfälle oder Beschwerden an den folgenden
autorisierten Vertreter von Midmark richten.
GULF DRUG LLC.
Al Barsha,
Dubai, VAE
Tel.: +971 4 501 4000
Fax: +971 4 501 4100
Kunden in Hongkong sollten alle Fragen, Vorfälle und Beschwerden an den unten aufgeführten autorisierten
Vertreter von Midmark richten.
Associated Medical Supplies Company LTD.
Raum 1201-1202, 12/F, Fo Tan Industrial Center
26-28 Au Pui Wan Street, Fo Tan, Shatin
New Territories, Hongkong
Telefon: +852 2604 9389
Fax: +852 2694 0866
Kunden in Qatar sollten alle Fragen, Vorfälle oder Beschwerden an den folgenden autorisierten Vertreter von Midmark
richten.
Tadmur Trading
Midmac Roundabout
Opposite Al Mana Petrol Station
Doha, Qatar
Tel: +974.44337000
Fax: +974.44337100
Kunden in Israel sollten alle Fragen, Vorfälle oder Beschwerden an den folgenden autorisierten Vertreter von Midmark
richten.
Medtechnica Ltd.
HaTnufa St 7
Petah Tikva, Israel
Tel: +972 3.925.4040
Fax: +972 3.924.9977

Deutsch - 6
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Katalognummer
Hersteller
Seriennummer
WARNUNG
Weist auf eine möglicherweise gefährliche Situation hin, die zu schwerwiegenden
Verletzungen führen kann.
Wichtige Informationen - Sicherheitssymbole
Vorsicht
Weist auf eine möglicherweise gefährliche Situation hin, die zu mittelschweren oder leichten
Verletzungen führen kann. Dieses Symbol kann auch auf unsichere Handlungsweisen
aufmerksam machen.
Gerätewarnung
Weist auf eine möglicherweise gefährliche Situation hin, die zu Sachschäden führen kann.
Zeigt die korrekte Oberseite
beim Versand an
Zerbrechlich
Trocken halten
Hinweis
Hebt Vorgänge, Verfahren oder Bedingungen hervor.
Bedeutung der Symbole
Medizinisches Gerät

Deutsch - 7
003-10309-99 © Midmark Corporation 2020
TP202 20-42-FO-00014 Rev A1 C2169
Bestimmungsgemäße Verwendung
Zum Stützen des Arms der Patienten während der Behandlungen an den oberen Extremitäten.
Sicherheitshinweise
WARNUNG
Änderungen an dieser Ausrüstung sind nicht zulässig.
WARNUNG
Nicht auf die Armlehne oder das Armlehnenpolster setzen.
WARNUNG
Der Stuhl darf nicht bedient werden, während dieses Zubehör installiert ist. Während einer
Behandlung keine Tasten drücken.
WARNUNG
Armlehne beim Aufstehen/Hinsetzen nicht als Grifffläche verwenden.
Anforderungen
Je nach Stuhlmodell muss möglicherweise eine rückseitig montierte Zubehörschiene installiert werden.
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
Sidan laddas...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
-
 53
53
-
 54
54
-
 55
55
-
 56
56
-
 57
57
-
 58
58
-
 59
59
-
 60
60
-
 61
61
-
 62
62
-
 63
63
-
 64
64
-
 65
65
-
 66
66
-
 67
67
-
 68
68
-
 69
69
-
 70
70
-
 71
71
-
 72
72
-
 73
73
-
 74
74
-
 75
75
-
 76
76
-
 77
77
-
 78
78
-
 79
79
-
 80
80
-
 81
81
-
 82
82
-
 83
83
-
 84
84
-
 85
85
-
 86
86
-
 87
87
-
 88
88
-
 89
89
-
 90
90
-
 91
91
-
 92
92
-
 93
93
-
 94
94
-
 95
95
-
 96
96
-
 97
97
-
 98
98
-
 99
99
-
 100
100
-
 101
101
-
 102
102
-
 103
103
-
 104
104
-
 105
105
-
 106
106
-
 107
107
-
 108
108
-
 109
109
-
 110
110
-
 111
111
-
 112
112
-
 113
113
-
 114
114
-
 115
115
-
 116
116
-
 117
117
-
 118
118
-
 119
119
-
 120
120
-
 121
121
-
 122
122
-
 123
123
-
 124
124
-
 125
125
-
 126
126
-
 127
127
-
 128
128
-
 129
129
-
 130
130
-
 131
131
-
 132
132
-
 133
133
-
 134
134
-
 135
135
-
 136
136
-
 137
137
-
 138
138
-
 139
139
-
 140
140
-
 141
141
-
 142
142
-
 143
143
-
 144
144
-
 145
145
-
 146
146
-
 147
147
-
 148
148
-
 149
149
-
 150
150
-
 151
151
-
 152
152
-
 153
153
-
 154
154
-
 155
155
-
 156
156
på andra språk
- italiano: Midmark 9A82001 Guida utente
- Deutsch: Midmark 9A82001 Benutzerhandbuch
- français: Midmark 9A82001 Mode d'emploi
- Türkçe: Midmark 9A82001 Kullanici rehberi
- dansk: Midmark 9A82001 Brugervejledning
- Nederlands: Midmark 9A82001 Gebruikershandleiding





























































































































































